|
||
|
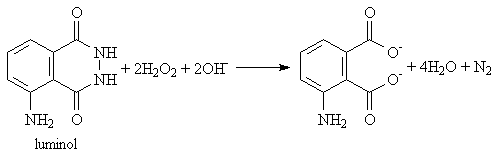
Luminol Description In the dark, liquids from two flasks are poured into a funnel connected to a transparent spiral tube. When the liquids mix, the solution glows bright bluish-green, and the glowing solution spirals down the tube. Explanation This reaction is very similar to the reaction that produces light in fireflies! One flask contains a solution of a compound called luminol, and the other contains an alkaline solution of an oxidizing agent, hydrogen peroxide (H2O2). (There are some other chemicals in these solutions as well, to make the reaction proceed quickly, but these are the key ingredients.) When the two solutions are mixed, the reaction produces a product which is in an excited state, that is, one of its electrons is in a higher energy state than it would like to be. When the electron drops down to a lower energy state, it emits a photon of light, and the solution glows. still have questions? email us! explanation furnished by UCCS's Dr. David Anderson
|
||