| Alka-Seltzer / Film Canister Rockets Description
A small amount of water and a piece of an Alka-Seltzer® tablet
are placed in a plastic film canister. The lid is quickly snapped on and the
canister turned upside down. Within a few seconds the canister explodes with a
loud pop, sending the bottom of the canister flying through the air.
Explanation
Alka-Seltzer and other effervescent tablets contain sodium bicarbonate (NaHCO3),
a base, and citric acid (C6H8O7), an acid.
(They also contain acetylsalicylic acid (aspirin) as a pain reliever, but it is
not involved in making the fizz.)
In the solid tablet the acid and base do not react, but when placed in water
the sodium bicarbonate reacts with the citric acid in an acid-base
neutralization reaction. One of the products is carbon dioxide gas, which causes
the fizz.
NaHCO3(aq) + C6H8O7(aq)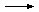 NaC6H7O7(aq)
+ H2O(l) + CO2(g) NaC6H7O7(aq)
+ H2O(l) + CO2(g)
How can I do it at home?
An adult must be present.
- Put a small amount of water into a film canister (Fuji film canisters work
better than Kodak)
- Put a small piece of an Alka-Seltzer® tablet in the film
canister.
- Put the lid on, turn the canister upside down and STEP BACK!!!
What would happen if...
- you tie a ribbon to the canister, can you figure out how high it flies?
- you try adding more or less water?
- you try adding more or less Alka-Seltzer®?
- you try adding hot water or cold water?
want to try some cool experiments with Alka-Seltzer?
Click here.
still have questions? email
us!
|