| Do a Red and
Green Chemistry Experiment at home! Materials Needed:
- a large glass or plastic cup
- water
- paper towels
- Celestial Seasonings Red Zinger tea (or any tea whose main ingredient is
hibiscus flowers will do)
- a base (such as ammonia, baking soda...)
- dish washing liquid
- dry ice (you can buy this at King Soopers or Wal Mart for $0.99/lb - you
need a minimum of 1 lb to do this experiment)
Preparation:
Make sure you're working with a parent during this experiment. Dry ice will
hurt your skin. ALWAYS wear heavy winter gloves when working with dry ice. Goggles are a good
idea too! Also make sure to do this experiment in the sink or somewhere you can
get messy.
Ready! Set! Experiment!
Step 1: Make about a half-cup of tea by simply putting a tea bag in
water in the cup.
Step 2: Very carefully pour some of your base (ammonia or baking soda
work well) into the tea.
Add enough such that the red tea turns green!
Why does this happen? Zinger tea contains hibiscus flowers, which are acid/base
indicators. The tea changes from red to
green to indicate you've added a base (the
baking soda or ammonia).
Step 3: Break the dry ice up into small pieces with a hammer,
or by dropping it on the floor inside a bag. Drop a
few small pieces of the dry ice into the green tea.
Watch closely as the tea turns back from green to
red. If the tea doesn't change colors after awhile,
add some more ice. Now, the tea looks nice and red again, but DON'T even THINK
about drinking it! You put a base in there, remember? Notice that the gas
released is still white and doesn't change as the liquid changes color.
What's going on here? When dry ice (solid carbon dioxide, CO2) is added, most of it
produces carbon dioxide gas, giving the bubbles, but some of it reacts with
water to form carbonic acid (H2CO3). The carbonic
acid reacts with the base to neutralize it, bringing it back to the red color
(sometimes the tea won't turn perfectly red again, if that happens, you can
always add an acid, like lemon juice, to get the red back)
CO2 + H2O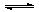 H2CO3 H2CO3
Step 4: And now for the mess... while the dry ice is bubbling through the tea
add a few drops of dishwashing liquid. You have created your own bubble machine!
As the dry ice goes from solid to gas phase in the water it's pushing the gases
through the tea and the soap to create bubbles.
Clean up:
Dump everything in the sink and rinse it well. All done.
More dry ice experiments and
information.
Still have questions?
Email us!

|