Unsociable Liquids
Introduction
You may have a bottle of Italian dressing in your refrigerator. Notice how
the oil and water don't mix? An how the oil floats on top of the water? Or you may have seen a
rainbow of colors on a puddle of water in the grocery store parking lot. That's
due to a thin layer of oil from automobiles floating on top of the water. Oil
and water don't mix because oil is insoluble in water, and it
floats on water because its density is less than that of water.
Safety
- Make sure you have an adult helping you.
- Do not drink any of the liquids!
- Do this experiment in a place where it's OK to make a mess.
Supplies
- 1/3 cup (80 mL) light corn syrup
- 1/3 cup (80 mL) water
- 1/3 cup (80 mL) cooking oil
- Food coloring - two colors
- 3 small glasses - paper cups will do
- 1 tall, narrow, clear glass or jar
- Funnel (optional)
- Various objects to test their densities, for example:
- small piece of carrot or celery
- small piece of candle wax
- small cork
- small glass marble
- small piece of metal like a BB or metal marble
- whatever else you might like to try
Directions
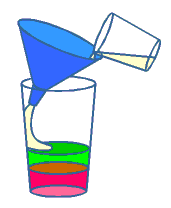
- Measure out the liquids into the small glasses.
- Add a few drops of one food coloring to the corn syrup and stir well. Add
a few drops of another color to the water and stir. Do not add any food
coloring to the oil.
- Pour the colored corn syrup into the tall, clear glass. Try not to
get it on the sides of the glass.
- Carefully pour the colored water down the inside of the glass to avoid
disturbing the corn syrup. You may wish to use a funnel to do this.
- Repeat step 4 with the oil.
- Carefully drop one or more of the objects into the liquids, and see what
happens!
Observations
- What happens as each liquid is poured into the glass?
- What happens when various objects are dropped into the liquids? Where do
they end up?
- What do you think would happen if you poured the liquids into the glass in
a different order? Give it a try!
What's Happening?!?
- Separate layers: You probably noticed that the liquids did not mix,
but remained as separate layers. The corn syrup and water do not mix simply
because the corn syrup is so thick. If you stirred them for a while, they
would mix, because corn syrup is soluble in water. The oil does not
mix with the water because it is insoluble in water. You see that in
Italian dressing and in oil on puddles of water.
- Top or bottom?: You used the same volume of each of the
liquids, but each has a different mass. The ratio of the mass of a
liquid to its volume is called its density. The corn syrup is more
dense than the water and oil, so it stays on the bottom. Water is less dense
than corn syrup, so it floats on top of it. Finally, the oil is the least
dense liquid of them all, so it floats on top of the water.
- Objects: Where did the various objects end up? Cork is less dense
than any of the liquids, so it floats on the oil. A piece of wax is more
dense than oil but less dense than water, so it sinks through the oil and
floats on the water. A piece of carrot is more dense that water but less
dense than corn sytrup, so it floats on the corn syrup (you may have to give
it a little push through the water layer if it has some oil stuck to it).
Finally, a marble or piece of metal is more dense than any of the liquids
and goes all the way to the bottom. You can use this "density
column" to compare densities of all kinds of things!
still have questions? email
us!
|